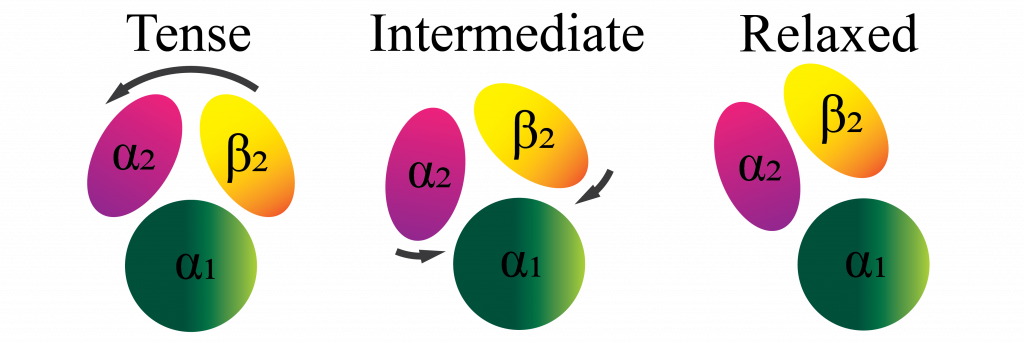
In sickle cell anaemia, the shape of red blood cells gets distorted due to the aggregation of mutant haemoglobin into fibrils inside the cells when the concentration of oxygen in the blood is low, and the haemoglobin molecule becomes tensed.
A new study by Dibyajyoti Maity and Debnath Pal at the Department of Computational and Data Sciences has shed light on the mechanism behind this aggregation, using molecular dynamics simulations. Haemoglobin is made up of two dimers, each of which has two subunits (α and β). The team has shown that the angle between the two dimers is a characteristic feature of the two states (tensed and relaxed) of the molecule, and demonstrated that both normal and mutant haemoglobin undergo tensed-to-relaxed transition, irrespective of the presence or absence of oxygen when starting from the tensed state. They also predict that an intermediate state between the tensed and relaxed states facilitates the transitions.
The mutation responsible for sickle cell anaemia causes a molecule called valine to replace glutamate.
The study also determined that in the mutated form of haemoglobin, it is the absence of glutamate rather than the presence of valine that plays a greater role in the formation of fibrils in sickle cells.