Drug delivery system that allows sustained release of drugs could help manage inflammation associated with this condition more effectively
Researchers at IISc have developed a microparticle formulation that allows sustained release of drugs to treat osteoarthritis, a chronic joint condition.
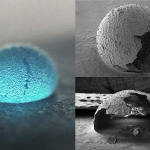
They have designed a polymer matrix made of poly (lactic-co-glycolic acid) or PLGA, an FDA-approved biomaterial, to encapsulate rapamycin, an immunosuppressant drug. Preliminary studies on cells cultured in the laboratory as well as in mice models have shown promising results indicating reduced inflammation and cartilage repair due to sustained drug release.
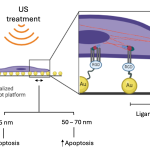
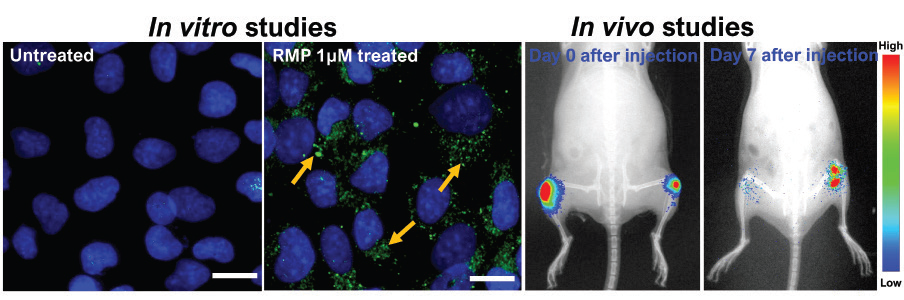
“In cell studies, rapamycin-loaded PLGA microparticles could release the drug for up to 21 days, and in animal studies, PLGA microparticles showed residence time up to 30 days after injecting the microparticles in the mice knee joint,” says Kaamini M Dhanabalan, PhD student at the Centre for BioSystems Science and Engineering (BSSE), IISc, and first author of the study published in the journal Biomaterials Science.
Osteoarthritis is associated with the wear and tear of the cartilage – the smooth tissue that protects bone joints – caused due to stress or aging. Existing treatment revolves more around managing pain and inflammation than treating the disease. Although several classes of drugs seemed promising in preclinical trials, low drug retention and rapid clearance from the target site have made clinical translation difficult.
The formulation developed by the IISc team, however, has a residence time of upto 30 days at the target site, with no evident signs that it may cause discomfort to patients. Such a sustained release system can improve patient compliance and reduce hospital visits.
PLGA is widely used for drug delivery applications and several drug formulations are currently used in clinics. Rapamycin is commonly used to suppress immune response in patients undergoing surgery for organ transplant to prevent organ rejection. Preclinical studies have shown its potential for treating osteoarthritis by preventing cell loss and cartilage damage, thereby reducing inflammation. However, the short drug retention time of about 1-4 hours demands frequent injections to maintain the therapeutic window in the joints.
Therefore, Dhanabalan and her colleagues combined the advantages of PLGA and rapamycin to create a system that would allow sustained release of the drug. This was achieved by encapsulating rapamycin in PLGA microparticles.
To evaluate the effectiveness of this formulation, chondrocytes or cartilage cells were cultured and subjected to various stresses to recreate osteoarthritis-like conditions under laboratory settings. This resulted in ailing chondrocytes with the hallmarks of osteoarthritis. These damaged chondrocytes recovered from osteoarthritis when treated with rapamycin-loaded PLGA microparticles.
“Preliminary studies using this newly-designed formulation can potentially reduce the frequent medical intervention to once a month. Detailed studies are in progress to explore the functional potential in mouse models of osteoarthritis,” says Rachit Agarwal, Assistant Professor at BSSE, and senior author of the study.