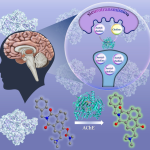
Using Cryo-EM, a powerful microscopy technique, researchers at IISc and collaborators have decoded the molecular architecture of a transporter protein controlling the movement of a key neurotransmitter.
Neurons or nerve cells communicate by releasing chemical signals called neurotransmitters. Each neurotransmitter can activate specific sets of proteins called receptors that in turn either excite or inhibit neural communication. A balance between excitation and inhibition is vital for the neural circuitry to maintain normal structure and function. Imbalances in excitatory or inhibitory inputs can result in disorders like seizures, anxiety, and schizophrenia.
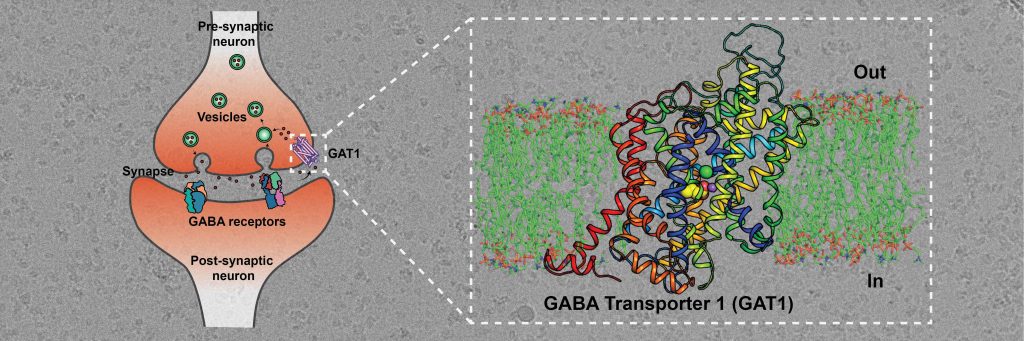
The inhibitory neurotransmitter Gamma-aminobutyric acid or GABA balances out the excitatory inputs from glutamate, another neurotransmitter. GABA-driven signaling at the neural synapses (junctions between neurons) is modulated by GABA receptor proteins that interact with GABA released from the preceding neurons in the circuit. Excess GABA released into the neural synapses needs to be recycled into neurons and surrounding glial cells for subsequent release events to happen. GABA transporters (GATs) are the primary molecules involved in this step – they employ sodium and chloride ions to move excess GABA back into the neurons. GATs are therefore vital molecules that orchestrate GABA signaling and function. They are an important target for the treatment of conditions like seizures.
The current study, led by Aravind Penmatsa, Associate Professor in the Molecular Biophysics Unit (MBU), deciphers the molecular architecture of GAT using cryo-electron microscopy. The technique has the capacity to image and reconstruct the structure of biomolecules that are more than a million times smaller than the width of a human hair.
The researchers purified GAT and used a novel approach to create an antibody site on this molecule. Antibodies help increase the mass of proteins and facilitate improved imaging through cryo-EM. The team was able to observe that the GAT structure was facing the cytosol – the inside of the cell – and was bound to a GABA molecule, sodium and chloride ions. This binding is one of many key steps in the GABA transport cycle. Deciphering it can provide vital insights into the mechanisms of GABA recognition and release into neurons.
The availability of high-resolution GAT structures is crucial for developing specific blockers of GABA uptake for treatment of epilepsies. It would also aid in studying how drugs prescribed to block GABA uptake interact with GATs.