Tracking changes at molecular interfaces is critical for designing new synthesis techniques and material systems. The nature of bonding at interfaces decides how the molecule reacts as well as the nature of the product formed from such reactions.
One complex yet interesting interface that has long been explored in theory and experiments is the gold-thiol interface (Au-S), owing to its importance in biosensing, nanomedicine, drug delivery, molecular electronics, molecular spintronics, molecular recognition, and noble metal nanoparticle catalysis. The Au-S bond formation involves different kinds of interatomic chemical (covalent and ionic bonding) and physical (van der Waals dispersion forces) interactions. The interplay of these interactions decides the structural, physicochemical, and spectroscopic properties of the Au-S interface. A simple description of the Au-S bond as either covalent or ionic fails to explain its properties and oversimplifies the complex bonding scenario.
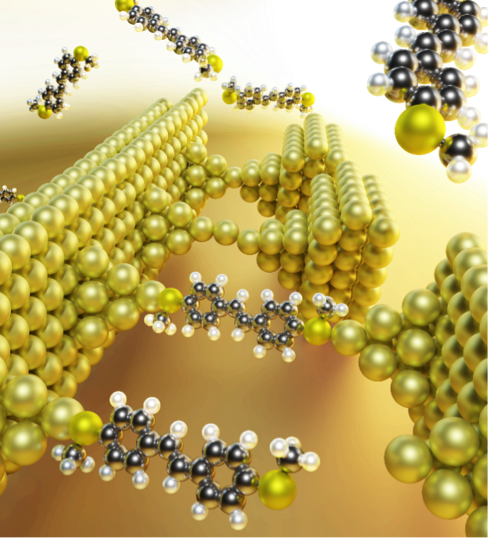
In a recent study published in the Journal of the American Chemical Society, Veerabhadrarao Kaliginedi’s team at the Department of Inorganic and Physical Chemistry (IPC) and their collaborators have developed a unique experimental approach to probe the nature of these complex interfaces. The authors include K Geetharani’s team at IISc, Keshaba N Parida’s team at IISER TVM and Joseph Hamill’s team at the University of Copenhagen.
The approach exploits the flicker noise generated in the single molecular conductance signal, which is induced by geometrical fluctuations at metal-molecule interfaces like Au-S when these interfaces are mechanically perturbed. As the fluctuations are induced by structural changes at the interfaces, the flicker noise induced by them directly correlates to the nature and thus the strength of the underlying interfacial interactions. Using their custom-built Mechanically Controllable Break Junction (MCBJ) setup, the authors quantified the flicker noise for a series of systems. They show that the flicker noise together with the single molecular conductance can be used to probe these complex interfaces at nanoscale. Measuring the flicker noise of molecular junctions with different conductance values but similar anchoring group chemistry can also help to identify similarities in binding.
As a proof of concept, the authors have shown for the first time how the interfacial electric field-catalysed ring-opening reaction of cyclic thioether unfolds under mild environmental conditions, which otherwise requires harsh chemical conditions for cleavage of the C-S bond. This experimental strategy can thus be exploited to probe reaction dynamics and study the mechanism of electrostatic catalysis reactions at a single molecular level.
This work has been highlighted on the cover page of JACS, Volume 146, Issue 13.