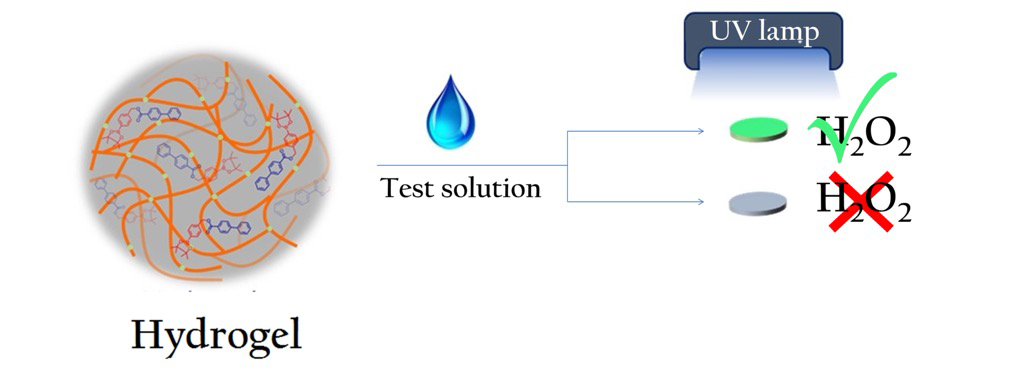
A paper-based sensor developed by IISc researchers can help detect even tiny volumes of hydrogen peroxide. This chemical is used widely in household and healthcare products like hand sanitiser as a disinfectant, in rocket fuel as a propellant, and is also found in biological cells.
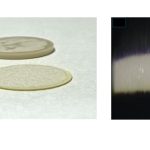
The technique they used involves preparing a gel from a solution containing a specially designed molecule, treated with a liquid that has hydrogen peroxide, and air-drying them on a thin paper disc about 0.45 cm in diameter. The paper disc emits green light when placed under a UV lamp, only in the presence of hydrogen peroxide. The intensity of the light was found to be directly proportional to the concentration of hydrogen peroxide.
“You can actually visualise this green emission (photoluminescence) with the naked eye. You don’t need any sophisticated instruments. All you need is a simple UV light source,” explains Arnab Dutta, PhD student in the Department of Organic Chemistry and first author of the study published in ACS Sensors.
Because the paper disc is low-cost, biodegradable and easy to use, it could serve as a powerful tool in low-resource settings, even for testing biological fluids like blood. Detecting hydrogen peroxide efficiently is also crucial in other fields; peroxide-based explosives, for example, can be traced using hydrogen peroxide which is sometimes used as a starting material.
When the researchers used their technique to randomly test five different hand sanitiser brands, they found that only three of them contained the level of hydrogen peroxide mandated by the World Health Organisation – 0.125%. A fourth appeared to have much lower than 0.125% and one had almost zero hydrogen peroxide.
“Hydrogen peroxide can be detected on a larger scale using titration and other experiments, but those are cumbersome and require training. This method is easy because of its simplicity,” says Uday Maitra, Professor in the Department of Organic Chemistry and senior author of the study.
Maitra’s lab has been working on developing several ‘sensitiser’ molecules that turn on the photoluminescence of elements called lanthanides in the presence of specific chemicals or compounds. They have previously developed paper-based sensors for detecting specific antioxidants in green tea – and thereby testing its quality – as well as sensors for various enzymes.
The sensitiser molecule they designed in this study enables a metal called terbium to emit green light under a UV lamp. When the sensitiser is combined with a masking agent, the green light vanishes. When hydrogen peroxide is added to this combination, it unmasks the sensitiser molecule, making it glow green once again. “The way we designed the mask … it is very specifically unmasked by hydrogen peroxide,” says Maitra.
Currently, the team is working on cutting down the reaction time; it takes a bit longer if the concentration of hydrogen peroxide is lower. Maitra adds that they are also working on developing a small portable device where the detection can be done in a more automated manner. “We are in touch with a start-up company in Chennai. We have a few prototypes made with UV LEDs and a camera, to generate the emission, take a photograph, and use an image processing app to quantify the amount of hydrogen peroxide.”