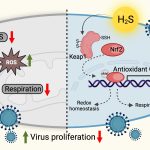
Researchers at IISc and their collaborators have identified a key role played by hydrogen sulphide (H2S) gas in suppressing the Human Immunodeficiency Virus (HIV). Increased H2S was found to have a direct effect on reducing the rate at which the virus multiplies in HIV-infected human immune cells. The finding paves the way for developing a more comprehensive antiretroviral therapy against HIV.
The team included researchers from the Department of Microbiology and Cell Biology (MCB) and the Centre for Infectious Disease Research (CIDR) at IISc, along with collaborators from the Bangalore Medical College and Research Institute. The results are published in the journal eLife.
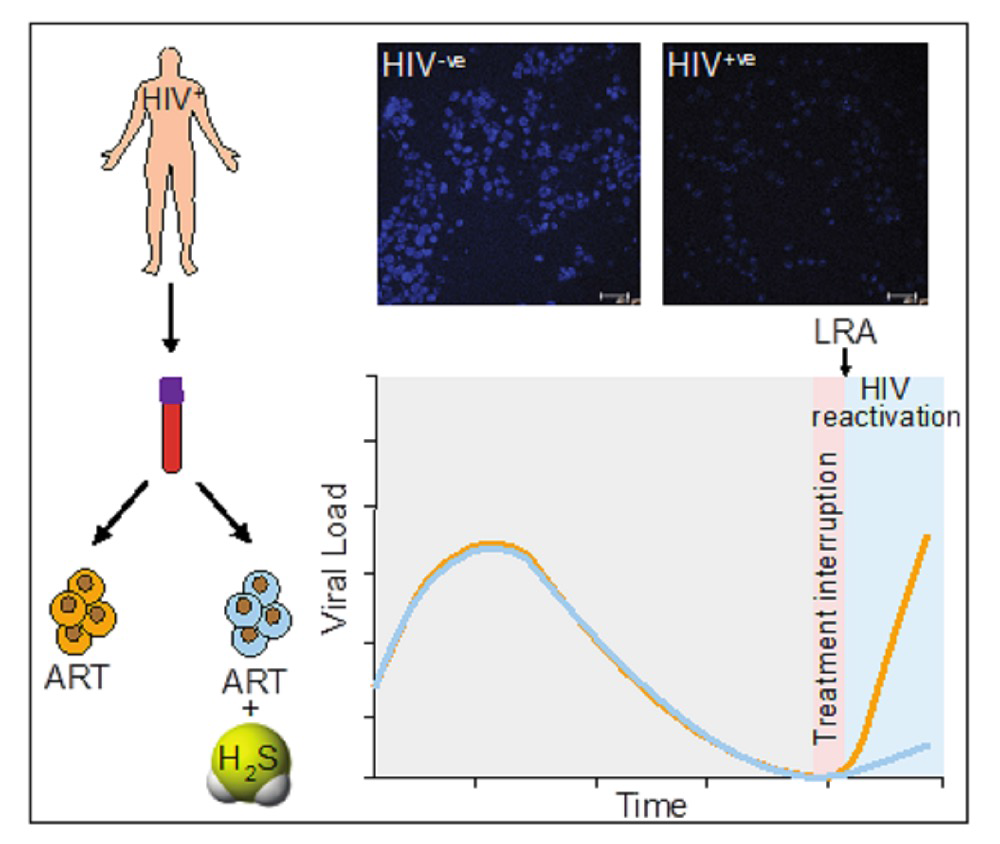
Current state-of-the-art combined antiretroviral therapy (cART) is not a cure for HIV. It can only suppress the virus and cause it to become latent. Unfortunately, in some cases, cART is known to fail even when patients follow their drug regimen fully. Certain negative effects are also associated with cART, such as the build-up of toxic molecules leading to ‘oxidative stress’ and loss of function in the mitochondria, the cell’s powerhouse. These effects can contribute to inflammation and organ damage. Stopping cART is also not an option because the virus can reactivate – emerge from its latent state – in the absence of therapy.
Scientists have recently begun exploring the beneficial effects of the presence of H2S in HIV-infected cells on both oxidative stress and mitochondrial dysfunction, according to Amit Singh, Associate Professor in MCB/CIDR and corresponding author of this study.
In a previous study, Singh’s lab developed a tool to measure oxidative stress in cells infected with HIV. “In that work, we showed that the chemical agent N-acetylcysteine was able to suppress HIV reactivation from latently infected cells,” he explains. “A German study later showed that N-acetylcysteine partly acts by releasing H2S molecules, which is when we began examining its role.” Previous work from Singh’s lab has also looked at the effects of counteracting oxidative stress by an antioxidant nanozyme during HIV infection. “Since H2S also functions as an antioxidant molecule, we wished to see whether our prior insights on oxidative stress and HIV could be translated to show the contribution of H2S on HIV infection.”
As the role of H2S in HIV has not been explored before, the authors had to set experiments up from scratch. “Studying the effects of a gaseous molecule on HIV required us to build and validate new model systems,” says Virender Kumar Pal, a PhD student in MCB and the first author of this study. “We started with experiments on established cell lines before moving on to cells donated by HIV patients in 2019. Our collaborators in the Bangalore Medical College and Research Institute, and Prof Annapurna Vyakarnam’s group at CIDR were of great help.” Detecting H2S inside the cells was also not a straightforward task. “Since H2S cannot be detected using conventional biochemical techniques, we had to use colorimetric and fluorometric techniques,” he recalls.
The researchers studied the effects of natural generation of H2S in HIV-infected cells as well as supplementing this with a chemical donor. “We observed a direct effect of H2S on suppressing HIV reactivation and replication along with all its other beneficial effects, such as maintenance of mitochondrial health and dissipation of oxidative stress in our [cellular] models,” says Singh. “Our results suggest that maintenance of HIV latency and reactivation are closely linked to the H2S levels in infected cells.”
Singh adds, “This opens the door to supplementing cART with chemical donors of H2S to lock HIV in a state of deep latency, potentially improving the lives of millions infected with the virus. Since H2S donors are already undergoing clinical trials for other diseases, they can quickly be repurposed for HIV treatment.”