Despite being portrayed largely as killers, these microscopic forms have beneficial roles too
(Image: Shyamili Gautam)
Almost a decade before the COVID-19 pandemic first emerged, a 2011 movie called Contagion explored the idea of what would happen to the world if a highly contagious virus transmitted by respiratory droplets and fomites spread quickly and killed thousands of people. It’s not the first time that viruses have been portrayed in fiction as the “villain”, and certainly may not be the last, especially after the past few years. But are viruses all bad?
Microscopic creatures that straddle the line between living and dead, viruses have been a part of our lives, literally, for millennia – researchers have found that our DNA may contain bits of ancient viral genetic material passed down over millions of years. While the benefits of these remnants are still being debated, scientists are increasingly convinced that viruses have a key role to play in helping our bodies fight diseases, not just cause them.
Viruses can help build vehicles that can deliver life-saving drugs into our body or target and kill cancer cells. Many life-saving vaccines contain inactive or weakened forms of viruses, which when introduced into our body, prime our immune system to recognise and prepare for future infections. The development of such vaccines can be traced back to 1796, when Edward Jenner found that dairymaids who suffered from cowpox were protected from smallpox; he took fluids from their blisters – which contained the cowpox virus — and injected them into patients suffering from smallpox. His experiments mark the first attempt to use a virus to tackle an infectious disease. Today, there are more than 25 approved vaccines given all over the world as protection against debilitating diseases, including polio, smallpox and measles. Together, they have helped save the lives of millions.
There are three major types of viral vaccines. mRNA vaccines – thrust into the limelight during the pandemic – carry a copy of a viral genetic material that prompts our body to produce the viral protein. Viral vector vaccines, like the ones developed by Jenner, carry a weak or inactive form of the virus into our bodies. The third type, protein subunit vaccines, contain a carefully selected protein or part of a viral protein. All these vaccines can prompt our immune system to produce the necessary antibodies needed to fight future infections.
At IISc, scientists such as Raghavan Varadarajan, Professor at the Molecular Biophysics Unit, have been developing viral vaccines for diseases such as HIV, influenza and COVID-19. His lab, together with Mynvax, the startup he co-founded in 2017, and other collaborators have been working to develop improved influenza vaccines, and more recently, a heat-tolerant COVID-19 vaccine. For scientists like him, even though the initial wet lab experiments and research can happen quickly, taking them forward to clinical trials is a much longer and more difficult process, he explains.
Another area where researchers have found viruses useful is targeting and killing cancer cells. An oncolytic virus is a virus that can selectively infect a cancer cell and force it to make multiple copies of the virus, until the cell bursts open and dies, releasing more viral antigens into the tumour environment. In 2015, T-VEC or OncoVex, became the first oncolytic virus to get approval in the USA and European Union as a “virotherapy” for treating advanced melanoma (skin cancer). Shashank Tripathi, Assistant Professor at the Centre for Infectious Disease Research (CIDR), IISc, and his team are working on developing an oncolytic Zika virus that can be used to treat neuronal and gonadal cancer. “Acute infection-causing viruses try to kill the infected cancer cell first, and they also try to activate the immune system. So, if you engineer the viruses to enhance these two properties, they will try to kill the tumor cells, recruit the immune cells at the site, and augment their function,” he explains.
A third application of viruses lies in the domain of genetic engineering. Each virus particle has a protein envelope that contains either RNA or DNA as the genetic material. Even with this simple structure, they possess the remarkable ability to replicate themselves quickly inside the host. Exploiting this ability, scientists have been able to transform viruses into vehicles called vectors that can deliver molecules, even specific genes, inside cells. Compared to other delivery methods, using viral vectors offers the advantage of delivering the cargo to all the target cells while causing minimal harm to the body.
Viral vectors are used to deliver either genes or short hairpin RNA into a host cell to produce specific proteins or to silence gene function, respectively. These vectors are often used to investigate the protein or gene’s function. Unlike other methods of gene delivery, viral vectors are less expensive and allow scientists to precisely control the output – what protein needs to be produced and how much – by controlling the number of viral particles that enter the host cell, explains Subba Rao Gangi Setty, Professor in the Department of Microbiology and Cell Biology, IISc, who works on vesicular transport inside cells. “Viral methods are much more advantageous because you get a large amount of – and uniform – gene expression; you can store viruses, reuse them, and still, you can have the same results with the same batch of viruses,” he adds.
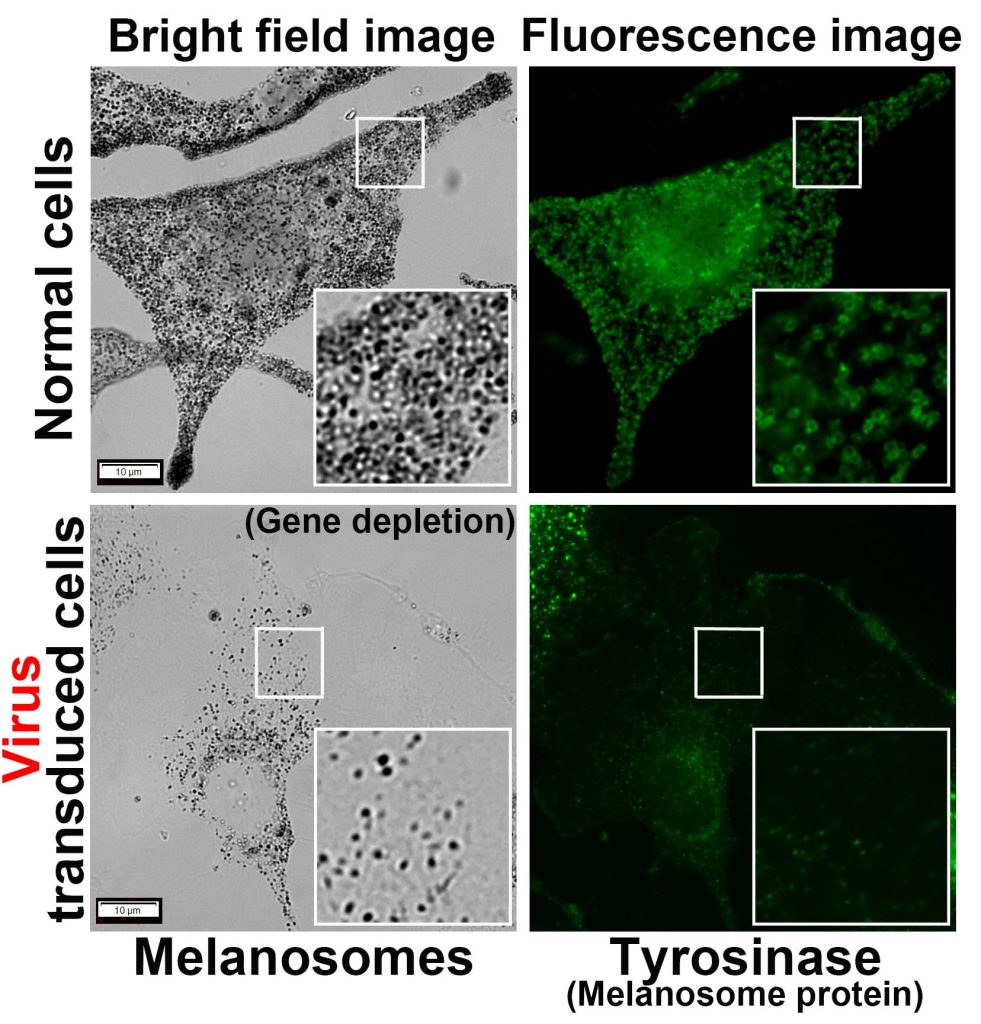
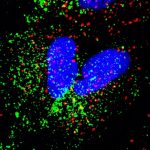
Viruses can also be used to reduce or shut down the action of a gene inside a cell, to pinpoint its function or investigate its role in diseases. The process involves modifying the virus to carry small pieces of genetic material, which when introduced into the target cells, seek out and block the target gene, “silencing” it. Kailash Chandra, a PhD student in the lab of Annapoorni Rangarajan, Professor in the Department of Developmental Biology and Genetics (DBG), says, “Our lab uses adenoviruses and lentiviruses for versatile purposes, including genetic knockdown and knockout.” Shyamili Goutham, PhD student in the lab of Ramray Bhat, Associate Professor in DBG, explains that the advantage of using viral vectors is that their effect on genes is consistent and efficient – because of their infectious nature – unlike other methods that usually lead to only temporary changes in gene expression. This is especially useful when scientists like her are keen on seeing these genetic changes pass down to subsequent generations of cells and studying their effects.
Apart from genetic engineering, cancer treatment, and vaccine development, viruses are also useful for other investigations and life-saving treatments. For instance, the use of bacteriophages – viruses that infect bacteria – as a model allowed scientists to identify DNA as our genetic material. Bacteriophages also find application in treating bacterial infections. The well-known CRISPR-Cas9 gene editing tool was inspired by the observation of viral gene insertions in bacteria. Many commercially available vectors, enzymes, and reagents utilised in research studies are derived from viruses. “Viral research from the beginning has provided a lot of reagents, which are continued even today,” says Shashank. “The study of viruses has provided fundamental principles and resources for the molecular biology field.”

(Photo courtesy: Kailash Chandra)