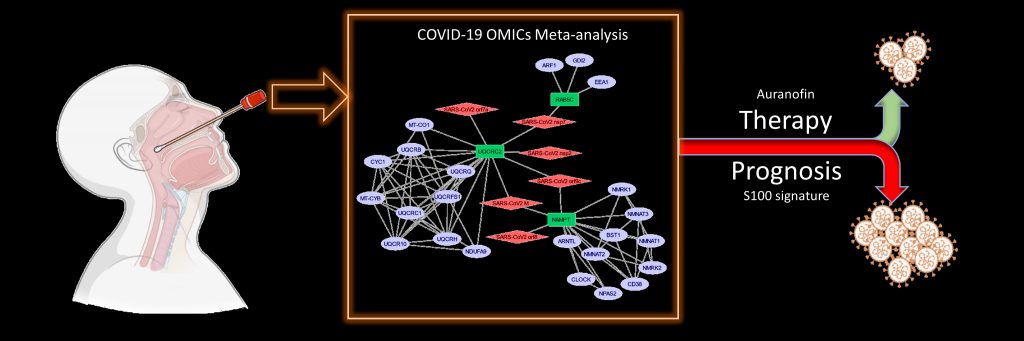
A recent study, led by Shashank Tripathi from the Centre for Infectious Diseases Research, reports two novel findings: a specific gene signature in nasal swabs which can predict COVID-19 severity, and the potential offered by an FDA-approved drug (Auranofin) for COVID-19 therapy.
In the study, the researchers analysed COVID-19 data from nasopharyngeal samples and were able to identify specific genes belonging to the S100 family which could serve as prognostic markers of severe COVID-19. This gene signature can be detected by RT-PCR in the nasal swabs which are collected for COVID-19 diagnosis.
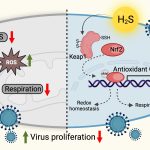
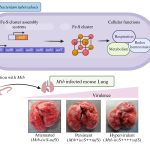
The researchers also identified multiple host processes which may be involved in virus replication and disease progression, and may serve as targets for host-directed therapy. Crucially, a redox regulatory protein called Thioredoxin (TXN) was found to be consistently overexpressed in COVID-19 patients. Auranofin, an FDA-approved drug that targets the enzyme thioredoxin reductase and blocks the thioredoxin pathway, was found to reduce SARS-CoV-2 replication in cell culture as well as in the hamster model. Auranofin is a safe and economical drug used for arthritis treatment. The study, therefore, suggests that it could serve as a promising COVID-19 antiviral.