Indoles and compounds derived from indoles are found in many natural substances. These compounds have extensive applications in pharmaceuticals and agrochemicals.
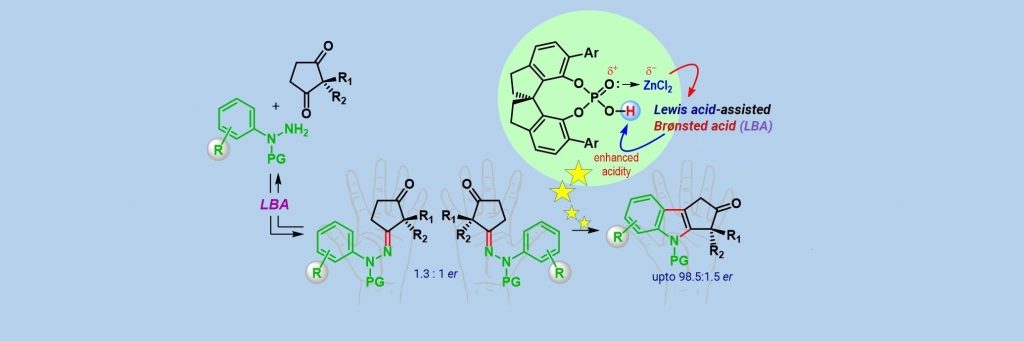
A research team from the Department of Organic Chemistry led by Santanu Mukherjee has now developed an efficient method to synthesise cyclopenta[b] indolones – an important class of indole derivatives. These molecules are ‘chiral’ in nature which means they can exist in two distinct forms whose 3D structures are non-superimposable mirror images of each other, called enantiomers. Often, only one enantiomer is biologically relevant, but it is extremely difficult to selectively synthesise just one of them individually in the lab – most existing methods produce a mixture of both.
The IISc team has overcome this hurdle using a catalyst which is also chiral in nature, and by carrying out a modified version of a century-old reaction called Fischer indolization. Computational studies through Density Functional Theory (DFT) calculations, led by Garima Jindal, helped in shedding light on the mechanism of this reaction.
Using this method, the team synthesised a range of enantioenriched cyclopenta[b] indolones which are challenging to make. Their method, therefore, increases the scope of making indole derivatives.