Tanweer Hussain’s lab seeks to decode one of biology’s most fundamental processes

One of the most memorable processes that we learn about in high school is protein synthesis – the “central dogma” of life. Proteins are essential biomolecules in our bodies, and the recipe to make them is encoded in messenger RNA (mRNA), which is produced from DNA via a process called transcription. Ribosomes, like a skilled chef, read these recipes, and instruct transfer RNA (tRNA) molecules, that, like obedient assistants, string the ingredients – amino acids – together, one by one.
“In terms of the general protein synthesis pathway, we might have learned the overall process. But there are new surprises still being uncovered, and we do not have a detailed understanding of how this process is regulated,” says Tanweer Hussain, who studies the critical initial steps of this pathway.
An associate professor at the Department of Developmental Biology and Genetics (DBG), Tanweer became intrigued by protein synthesis during his PhD years.
With a background in biochemistry, he did his graduation and post-graduation at Aligarh Muslim University and went on to do a PhD with Rajan Sankaranarayanan at the CSIR-Centre for Cellular and Molecular Biology (CCMB), Hyderabad. His PhD project focused on understanding how proteins are accurately synthesised in a cell with the help of an enzyme called aminoacyl-tRNA synthetase.
During this time, he also got a chance to discuss his PhD work with Venkatraman (Venki) Ramakrishnan, who was visiting CCMB. At that time, the latter hadn’t yet received the Nobel Prize, but his work on ribosomes was well known. Inspired by his work, Tanweer decided to apply for a postdoctoral position in Venki’s lab at the MRC Laboratory of Molecular Biology in Cambridge, UK.
Because of delays in some experiments, his PhD got extended and he couldn’t join Venki’s lab when he had originally planned. By the time he joined in 2011, Venki had won the Nobel Prize, and Tanweer became his first postdoc hire after the award.
“He was extremely busy at the time I went there. He also took up the additional charge of being the President of the Royal Society, so he used to spend three days in London and only two days in the lab (in Cambridge),” remembers Tanweer. “Despite his busy schedule, he would interact with lab members on a regular basis. It was a great experience to learn how he picks up a problem and approach solving it.”
Peering at proteins
The first problem that Tanweer picked was decoding the structure of the preinitiation complex (PIC) in yeast. Revisiting our cooking analogy, the PIC is like a team of helper proteins and RNA which gathers all the required ingredients and the right tools before protein synthesis begins.
Scientists conceptually knew what the PIC does, but the structure and mechanistic details weren’t fully clear. Tanweer’s team wanted to understand how the PIC components fit together, like pieces of a jigsaw puzzle.
For this, they used a technique called X-ray crystallography. In this method, protein complexes are crystallised into tiny, orderly crystals, and then X-rays are passed through them. The X-rays scatter when they hit atoms in the protein, creating a distinct pattern. By analysing this pattern, researchers can piece together a detailed 3D structure of the PIC.
However, try as he might, he couldn’t obtain crystals of these protein complexes. Meanwhile, advances in cryo-electron microscopy (cryo-EM) at the MRC offered a promising alternative, so he switched to that. In this technique, the complex is flash-frozen and visualised under a transmission electron microscope. An electron beam passes through the sample and produces patterns that can be used to decipher the structure of the PIC.
Although he was able to decode most pieces of the puzzle, there was still a missing link that he couldn’t decipher, a protein complex named eIF4. The function of this protein complex is to bring along the mRNA when the entire PIC setup is ready. Imagine the PIC as a car, then eIF4 complex would be the base of a car key, and the actual key would be the mRNA. The role of the eIF4 proteins is to help insert the key (mRNA) into the socket, which in this case is the ribosome and the PIC.
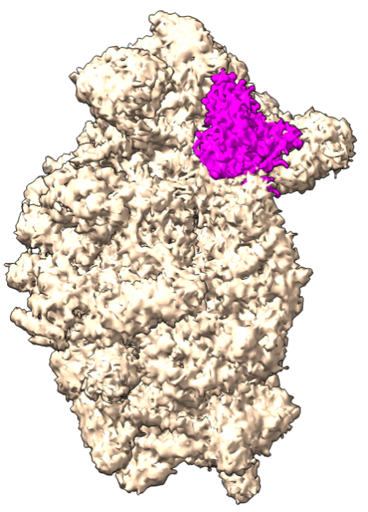
Tanweer’s quest to decode the eIF4 complex structure continued when he joined IISc because these proteins are not just required for initiation but also regulation of protein synthesis, which is an overarching theme of his lab. “Till today, no one has figured out the structure of the entire eIF4 complex,” Tanweer says. “No one knows what it looks like and how it activates mRNA.” His group aims to understand the molecular mechanisms involved in the initial steps of protein synthesis and its regulation, using biochemical and structural approaches.
Chasing after checkpoints
All proteins have unique functions, and so they are not required at all times. Therefore, it is important to stop the synthesis of a protein when not needed, by denying its mRNA access to the ribosomes or by other means. In addition, if we understand how mRNA is recruited to the PIC, it will help us understand how mRNA access is blocked. Tanweer’s team has been able to pinpoint one such factor called the eIF4B protein, which helps in bringing mRNA to the ribosome.
Such deep insights into protein synthesis also paid off during the COVID-19 pandemic. When his team delved into dynamics of SARS-CoV-2 proteins, they found a way to inactivate a viral protein called nsp1 that binds to the ribosome in the host cell and blocks the entry of mRNA, shutting down essential protein production.
In collaboration with Sandeep Eswarappa’s lab at the Department of Biochemistry and Shashank Tripathi’s group from the Centre for Infectious Disease Research, his team deciphered the structure of a drug bound to the viral nsp1 protein using a computational approach. Using this information, they found that an existing drug called montelukast – used for asthma treatment – is capable of binding to nsp1 and blocking this viral protein, and restoring protein synthesis.

Post-COVID-19, his lab is continuing to explore how viruses manipulate the protein synthesis machinery in host cells. As one of their new model systems, they started studying plant viruses.
“We want to understand how these viruses target plant ribosomes. If we know more about the plant system, perhaps we can address issues of pest control and fungal diseases that affect a lot of agricultural crops,” says Tanweer.
Studying protein synthesis is not easy. Running each sample in cryo-EM, for example, can cost thousands of rupees. Additionally, the sample itself isn’t always “clean” or perfect. Since the protein complex needs to be frozen before imaging, a few elements of those complexes shunt off sometimes, necessitating repeated sample preparations.
“When you give your best shot and you still don’t get the results, you can get depressed and demotivated,” he adds. “But hard work and perseverance are what you need. When we fail, that failure still also teaches us what is going wrong or what is not working.”