Scientists at MBU are exploring the use of AI to decode protein folding, form, and function
“They cracked the code for proteins’ amazing structures.” This was the title of the press release announcing the 2024 Nobel Prize in chemistry. One half went to David Baker, Professor of Biochemistry at the University of Washington, for computational protein design, while Demis Hassabis and John Jumper, scientists at Google’s DeepMind, got the other half for their work on protein structure prediction using AlphaFold.
Scientists at the Molecular Biophysics Unit (MBU) at IISc are already using such AI tools to study biological structures. But David’s work particularly inspired Rishikesh Narayanan, Professor and Chair of MBU, to develop new ways of using existing AI tools in designing proteins with specific functions. “It could be an antibiotic, snake anti-venom or scorpion anti-venom,” says Rishikesh.
AlphaFold, for its part, has helped scientists solve a roughly 50 year-old problem – using a protein’s amino acid sequence, or its building blocks, to predict its final folded structure. Trained on a massive repository of protein structures in the Protein Database Bank (PDB), AlphaFold uses AI to go from a protein’s sequence to its structure in mere minutes.
Spurred by such possibilities, Rishikesh and other scientists at MBU are keen to build a stronger bridge between biophysics and AI research at IISc. Their ultimate goal is to collaborate with colleagues in engineering departments and build novel toolboxes to tackle specific biophysics problems, Rishikesh explains.
Biophysics is a good space to explore the use of AI because of the cleanly curated data and well-defined questions in certain areas of research, says Anand Srivastava, Associate Professor in MBU. Advancements in computational facilities have made computing power less of a limitation as well. “[The field] has a huge amount of untapped data that has come from physics-based experiments and theory,” he says. “A lot of [that] available data could be used to develop new AI models and make transformative advances in the field.”
Anand and his team have been trying to delve deeper into understanding how some of these AI algorithms work. They recently published a method to predict whether a given short peptide could be an antimicrobial. They first computationally modelled interactions within the peptide using molecular dynamic simulations. They then incorporated that information into a classical, convolutional neural network algorithm that is used to classify images. The result: a neural network that can analyse a short peptide and predict whether it can be used as an antimicrobial or not.
They are now extending this with advanced deep learning methods, like Graph Attention networks, to faithfully predict larger anti-microbial peptide candidates. “Eventually, what we want to do is not just have a black box where you give a sequence and it gives you an antimicrobial peptide, but we would also like to know what design features are making it antimicrobial,” says Anand. “And [the algorithm] should be robust enough to tell us what would be the mechanism of antimicrobial activity for a given sequence, instead of just saying that it is antimicrobial?”
He has also been working on metamorphic proteins, which contain the same amino acid sequence but fold into different structures for different functions. Proteins that manage our internal body clock – our circadian rhythms – are an example. Working with a large dataset on 57 different proteomes of different species, his lab is trying to predict what kind of sequences would lead to metamorphic proteins. This work is hosted online so that the community can make use of it as a design tool.
“This is fascinating from a molecular switch design point of view,” explains Anand. “Suppose you have a liquid and you want to know whether it is acidic or basic. If you have a protein sequence that folds in one confirmation in acidic media and another in basic media, then you can actually use it as a tool … because it switches in different environments.”
Unravelling viral proteins
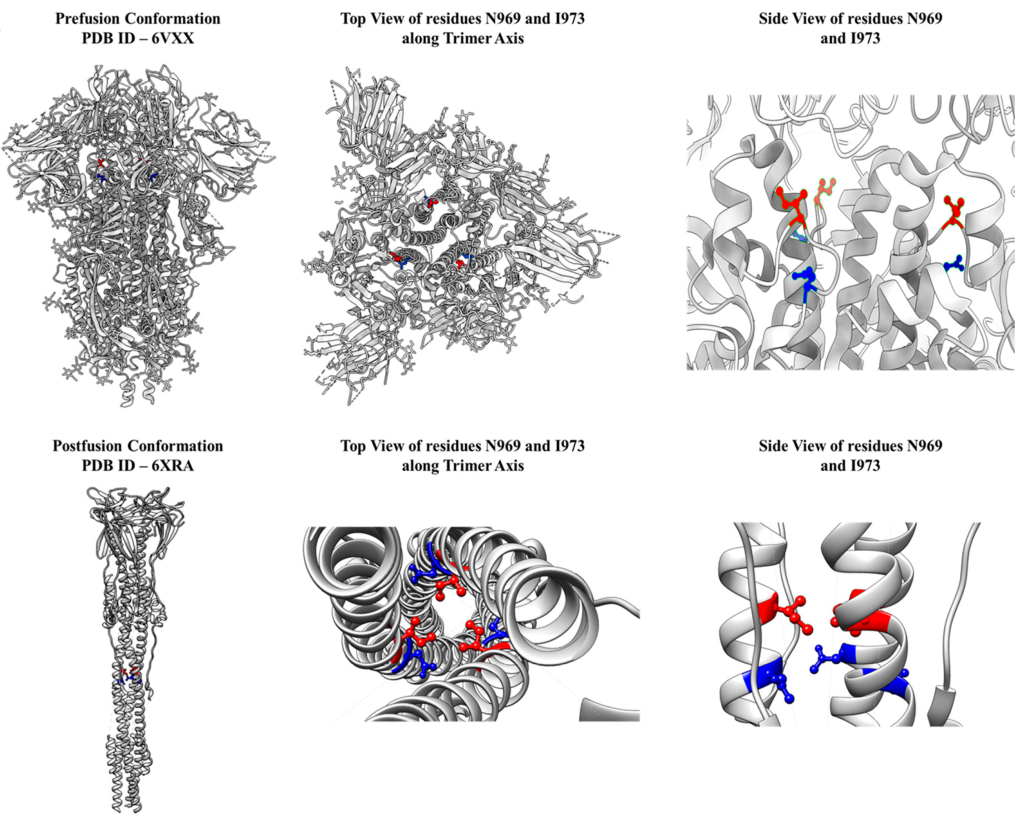
In parallel, Anand has been working with Raghavan Varadarajan, Professor at MBU, on vaccines against viral diseases. One of the first steps during an infection is the binding of viral surface proteins to receptors on cells in our body. Vaccines are usually made up of similar proteins – or RNA that encodes such proteins – to trigger the release of antibodies that can ward off future infections.
During the process of viral entry into the cell, one of the important viral surface proteins transforms from a less stable “pre-fusion” form to a more stable “post-fusion form” and the energy released helps the infection process. When used in vaccines, however, the protein may spontaneously switch to the more stable form.
“At best, this is useless because you don’t want antibodies to recognise the post-fusion confirmation – they won’t prevent infection – and at worst, some of these antibodies can actually enhance infection, by promoting viral entry into cells,” says Raghavan. “Therefore it’s important to be able to stabilise these proteins in their native conformation found on the viral surface.” Less stable versions of the proteins also tend to aggregate and lead to low commercial yields when mass-produced, Raghavan adds.
Raghavan and his lab are working on creating thousands of mutated versions of a protein or a group of proteins, and screening out the ones that are most stable. A dataset of these stable proteins can then be used to train AI predictors. Once one “machine learns” the principles that govern protein stability, says Anand, we can potentially use it to create a few targeted mutations that may lead to more stable proteins.
Vidya Mangala Prasad, Assistant Professor at MBU, also works on conformational changes in viral surface proteins, but her focus is on preventing those changes from happening. Surface proteins found on the dengue virus, for example, need to undergo specific conformational changes to fuse with cells in our body. “Generally if the virus cannot fuse with host cell membranes, it cannot establish infection,” she explains.
Her plan is to use AI tools to find small protein molecules that can bind to viral surface proteins and block the necessary conformational changes. If one knows the structure of the protein and the location where a blocker must bind to prevent its function, AI tools can help design this blocker.
“It’s [like] working backwards – knowing the target structure, we can determine what kind of complementary structure we need to block the target protein’s movement,” she explains. AI can then help us figure out the sequence that would lead to the desired folded structure and simulate its function computationally, thus narrowing down the final number of candidates that need lab testing.
Aravind Penmatsa, Associate Professor at MBU, is similarly interested in using AI to find therapeutic molecules that block protein function in neurons. His goal is to identify antibodies that could bind to and prevent the activity of specific neuronal receptors or transporters implicated in exacerbating conditions like epilepsy, pain, and anxiety.
Be it designing drugs, stabilising vaccines or studying protein folding, AI can help scientists push the boundaries of biology to design better therapeutics. “AI thrives in places where we have data and don’t have [enough] models, and biology is one of those fields,” says Chiranjib Bhattacharyya, Professor at the Department of Computer Science and Automation.
Rishikesh believes that combining the power of AI with biophysics techniques – cryo EM, peptide synthesis, NMR, and so on – can lead to major interdisciplinary breakthroughs. To this end, MBU has started several initiatives ranging from symposia and seminar series to journal clubs and study groups to boost awareness and collaborations. The department also plans to offer a new course on “AI in structural biophysics and molecular therapeutics.”
“AlphaFold and the David Baker-style interdisciplinary research come from people talking to each other – the biologists talking to the computational people and the computational people talking to the biologists,” Rishikesh adds.