For decades, the diversity in shape, size, and function of proteins has intrigued researchers who have tried to understand the forces that dictate protein structure and stability. Stability under thermal stress is an important factor in protein design; by altering the amino acid side chains, the stability of a protein can be changed.
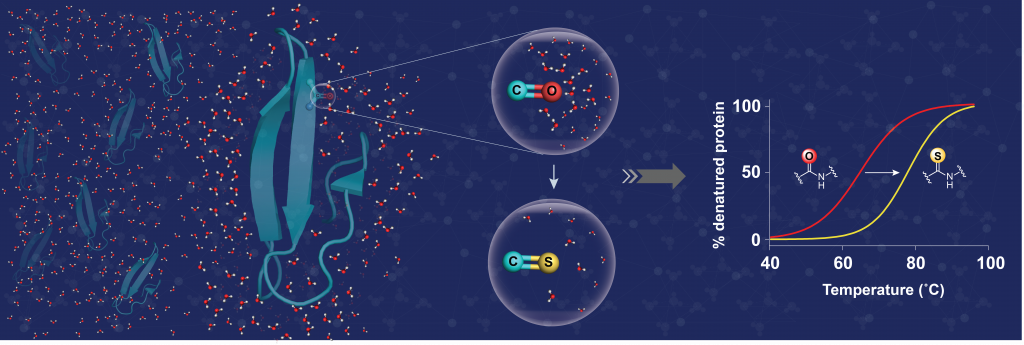
Now, researchers led by Jayanta Chatterjee at the Molecular Biophysics Unit report that the thermal stability of proteins can also be brought about by chemical modification of the polypeptide backbone. The backbone of a protein is composed of amide bonds, which are well solvated by (complexed with) the surrounding water. By replacing the oxygen atom of a single amide bond with sulphur, the researchers could significantly lower the solvation or interaction of the thioamide bond with water. This creates a lipophilic microenvironment that acts in synergy with neighbouring amino acids to significantly increase the protein’s stability.
The results not only highlight the utility of this single atom substitution to increase protein stability, but also the critical role of surrounding water in influencing protein stability. The marked desolvation of amide bonds also makes this tool attractive for the design of membrane-permeable
peptide drugs.