A new diagnostic approach to detect pathogenic bacteria uses Raman spectroscopy, a technique normally used to probe material structures
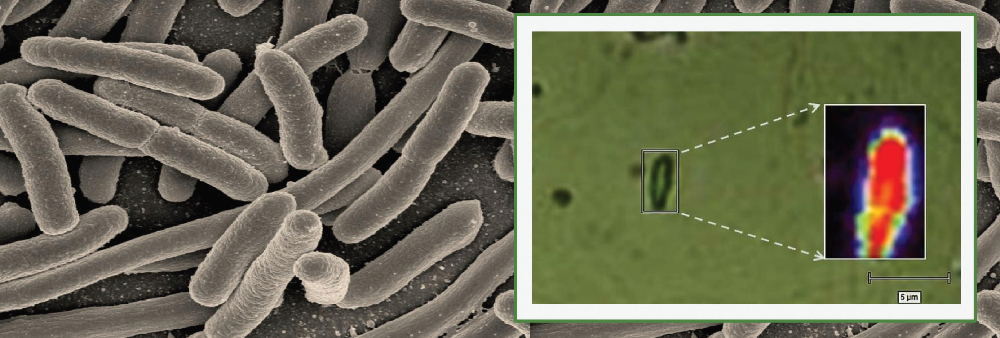
The key to treating infectious diseases lies in quickly detecting the pathogen or disease-causing agent, as well as checking for its viability – whether it is alive or dead in patient samples. Determining viability also helps physicians decide the dose of antibiotics to be prescribed, and reduces the possibility of overprescription that can lead to antibiotic resistance.
A research team at IISc has now developed a method to rapidly identify and check whether a disease-causing bacterium in a sample is alive or dead. It employs Raman spectroscopy – a technique that is usually used to identify chemical bonds in materials – to recognise bacteria and test for their viability.
“The uniqueness of this study is the rapidity and sensitivity of the method, and its potential for being modified into a bed-side, table-top device for diagnosis,” says Srividya Kumar, a former PhD student at the Department of Inorganic and Physical Chemistry, and first author of the study published in the journal Analytical and Bioanalytical Chemistry.
Infectious bacteria are usually identified by techniques that involve growing them on a nutrient medium in a Petri dish. However, it can take two to three days to track their growth and confirm whether they are dead or alive. In addition, bacteria that are difficult to grow in lab cultures often go undetected. More sophisticated methods such as Polymerase Chain Reaction (PCR) create a genetic profile of the bacterium, but cannot tell whether it is alive or dead.
Raman spectroscopy is used widely in the field of chemistry to probe the structure of molecules. In this study, however, Kumar and her colleagues combined this approach with advanced microscopy to check for the presence of bacteria.
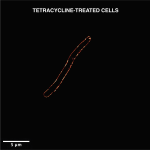
Using a microspectroscope, the researchers bounced a laser beam off the sample and captured the light scattered by it in the form of a spectrum, which varies with the biochemical composition of the bacterium. Each bacterial species generates a unique spectrum with specific values for intensity and wavelength position of the scattered light, depending on the type of chemical bonds present inside the bacterial cell.
The material that is used for mounting the bacteria in this technique, known as the substrate, is also crucial because some substrates can add noise to the biochemical profile. The researchers developed a neutral, aluminium-based thin film substrate which does not generate background signals that may interfere with the spectra.
The researchers used this technique to detect whether the tuberculosis bacterium was present in a sample medium that resembles sputum. They also tested the approach on samples containing five other microbes. They were not only able to differentiate between the various bacterial species, but also between living and dead bacterial cells – since the chemical composition of the two differs – within two to three hours after sample collection. The technique was sensitive enough to generate a spectrum even for a single bacterial cell.
As each bacterial species gives rise to a unique spectrum, it might be possible to eventually build a database of pathogens that can be used in clinical diagnosis.
“Though we have validated the method for tuberculosis, the methodology can be extended to any type of bacterial infection,” says Deepak Saini, Associate Professor in the Department of Molecular Reproduction, Development and Genetics, and one of the senior authors of the study.