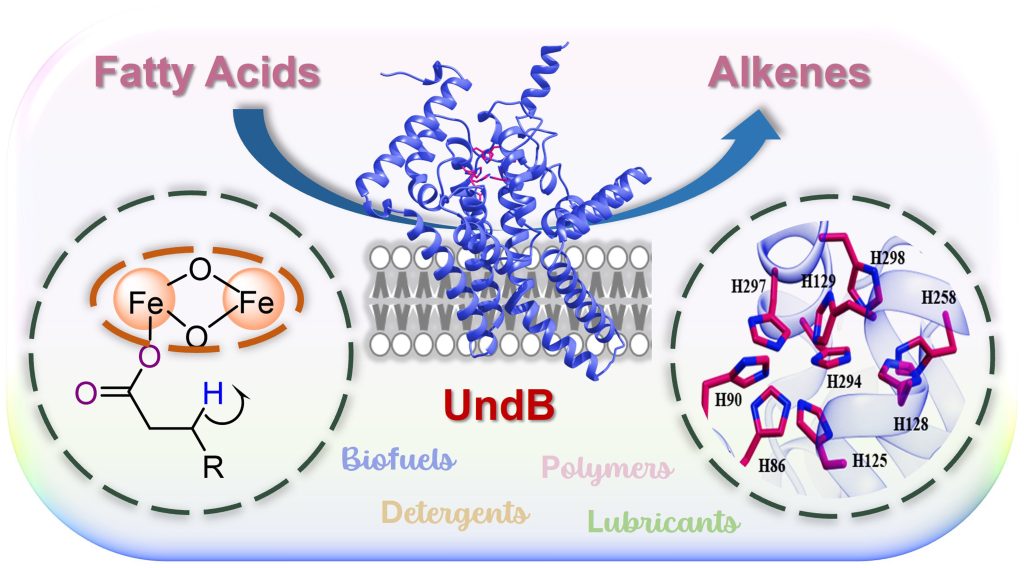
There is an increasing demand for the sustainable production of molecules called hydrocarbons because they are the major components of fossil fuels and have utility in polymer, lubricant, and detergent industries. Hydrocarbons like terminal alkenes (1-alkenes) produced biologically using the cells of living organisms (biosynthetically) can serve as the next generation of hydrocarbon-based biofuels.
Scientists have discovered a membrane-bound enzyme called UndB that can convert fatty acids, which are naturally highly abundant, to terminal alkenes. This enzyme is thought to be the fastest 1-alkene-producing enzyme known to date; however, the enzyme could not be studied so far because of its recalcitrant membrane-bound nature.
Researchers at the Department of Inorganic and Physical Chemistry (IPC), led by Debasis Das, have purified and characterised the challenging enzyme, UndB, found natively in the bacterium Pseudomonas mendocina. They have shown that UndB is a redox- and oxygen-dependent enzyme with a diiron center at its active site.
The researchers found that the enzyme works optimally at 25°C and catalyses the conversion of medium-length fatty acid substrates better than long or short-length substrates to produce corresponding 1-alkenes. Moreover, the researchers have deciphered the mechanism of action of this enzyme. The findings shed light on how UndB catalyses the chemically challenging conversion of fatty acids to terminal alkenes at the cell membrane interface, which can help develop novel biocatalysts.
Considering the ever-increasing energy demand, the global calls for sustainability, and the importance of hydrocarbon-based biofuels, the researchers believe that their findings will add momentum to the sustainable production of biofuels and green commodity chemicals.
According to the researchers, their findings can be translated for the highly efficient production of 1-alkenes with many biotechnological applications. Furthermore, the approach to investigating UndB can be extended to decipher many other enigmatic membrane-bound enzymes responsible for the biosynthesis of valuable chemicals in nature.