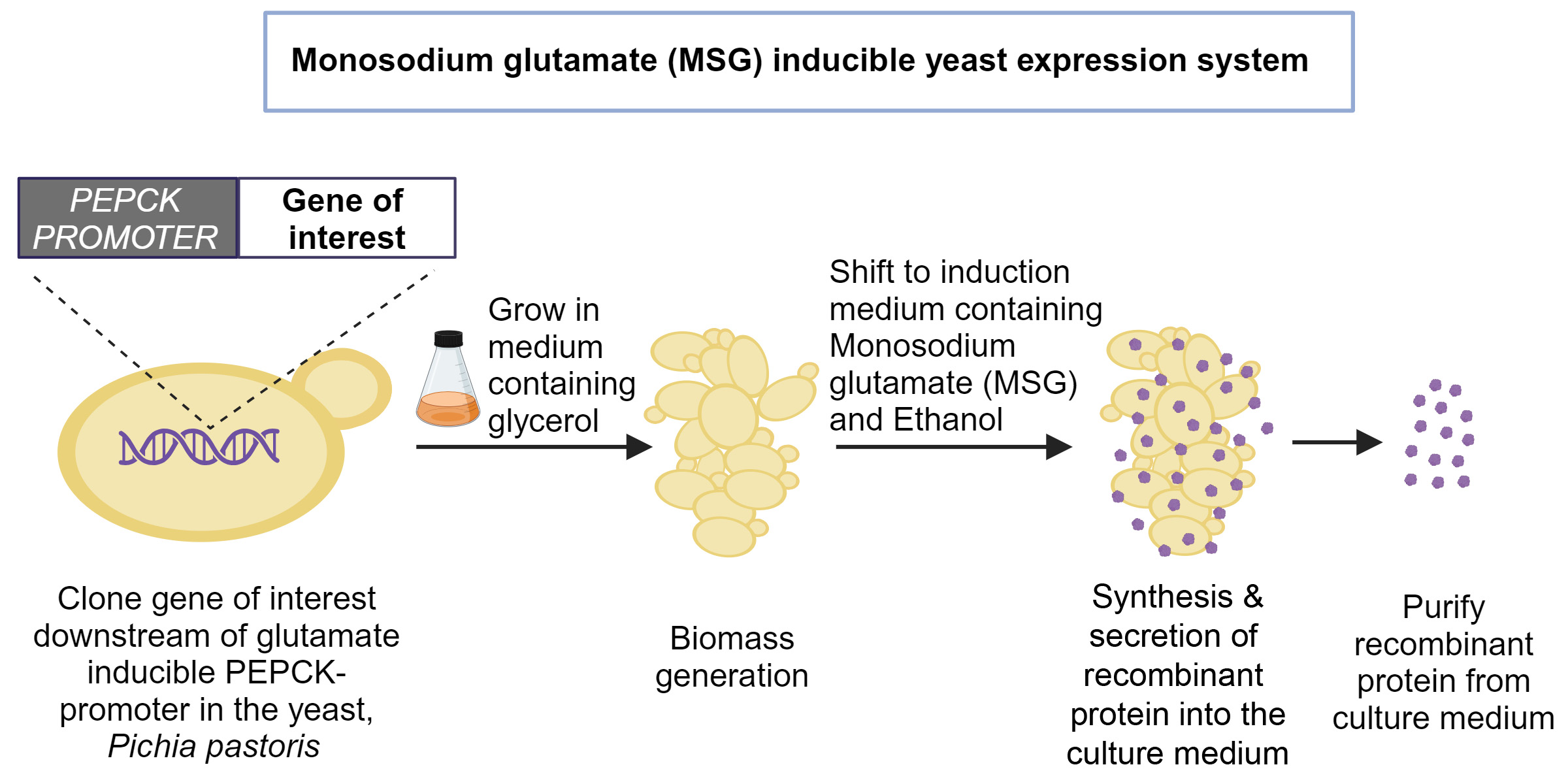
Mass production of recombinant proteins using yeast cell “factories” needs methanol, a compound that requires safe handling, carries the risk of catching fire, and sometimes produces harmful byproducts. Researchers at the Department of Biochemistry (BC), Indian Institute of Science (IISc), have now developed an alternative safer process that instead relies on a common food additive called mono-sodium glutamate (MSG).
Recombinant proteins, such as vaccine antigens, insulin and monoclonal antibodies, are mass-produced by growing modified bacterial, viral or mammalian cells in large bioreactors. The most widely used organism is the yeast Pichia pastoris (now called Komagataella phaffii). It contains a unique promoter – a specific gene region which can be activated by methanol. This promoter codes for an enzyme called alcohol oxidase (AOX).
To mass-produce a recombinant protein, the gene coding for that protein is spliced into the yeast genome right next to the AOX promoter. The yeast cells are then fed glycerol or glucose as the carbon source. Once enough cells have formed, methanol is added, which then activates the AOX promoter, and the cells start producing the recombinant protein in copious amounts.
Most industries use this methanol-induced process for producing recombinant proteins. However, methanol is highly flammable and hazardous, requiring stringent safety precautions, points out PN Rangarajan, Professor at BC and corresponding author of the study published in Microbial Cell Factories. Methanol also metabolises to form hydrogen peroxide which can induce oxidative stress in the yeast cells or damage the recombinant proteins.
To solve this problem, Trishna Dey, a former PhD student at BC, started looking for alternatives. After an extensive search, the team found that mono-sodium glutamate (MSG), a USFDA approved food additive, can activate a different promoter in the yeast genome that codes for an enzyme called phosphoenolpyruvate carboxykinase (PEPCK). Activating this promoter with MSG led to protein production similar to methanol activation of the AOX promoter.
Optimising the cell culture medium for this new and untested process was challenging, says Neetu Rajak, first author and PhD student at BC. For a long time, the yeast cells grew poorly in shake flasks and produced very little recombinant protein. “There was a time when we almost gave up because we thought it was not going to work,” recalls Rangarajan.

The group eventually figured out that using MSG alone was not enough. Vedanth Bellad and Yash Sharma, project assistants at BC and co-authors, explain that they tried supplementing the culture with various other compounds, until one finally did the trick: ethanol. Adding ethanol helped the cells grow faster, which increased the biomass and the amount of recombinant protein produced. Ethanol is also safer for yeast cells compared to methanol, as it does not produce toxic byproducts when broken down.
To test their process, the team tried producing the SARS-CoV-2 receptor binding domain – a widely-used vaccine antigen that has been successfully expressed in yeast and mammalian cells. They found that their new expression system produced twice the amount of antigen compared to the methanol-induced process.
The researchers hope that this novel and indigenous expression system can be used in biotech industries to mass-produce valuable proteins including milk and egg proteins, baby food supplements and nutraceuticals, apart from therapeutic molecules. An Indian patent has been granted for the expression system and a US patent has been filed. The team is also looking for industry collaborators to scale up the system for mass production.