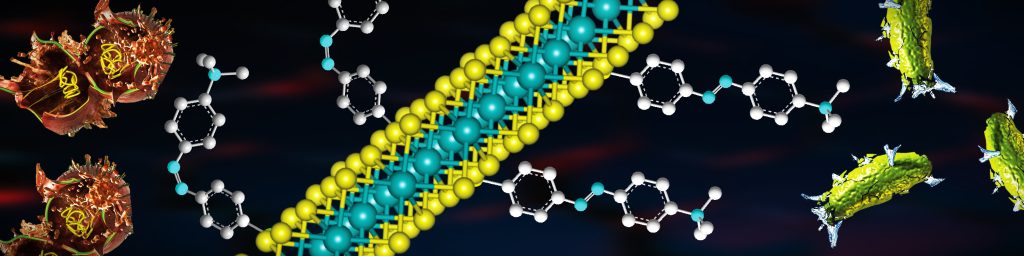
Bacterial infections making open wounds worse is becoming increasingly common. To aid in the treatment of festering wounds and to combat selective bacterial infections that are antibiotic-resistant, researchers at IISc have developed a strain-specific bactericidal nanomaterial with a photochemical property. The material can especially help combat Gram-positive or Gram-negative bacteria, like the Methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa, which cause difficult-to-treat infections.
The study, authored by Jagabandhu Sahoo, Soumyashree Sahoo, Yogeswari Subramaniam, Preeti Bhatt, Subinoy Rana and Mrinmoy De, was published in Angewandte Chemie.
The key feature of this technique is that the bactericidal materials do not rely on antibiotics. The researchers succeeded in producing a UV and visible light-responsive nanomaterial that can target either Gram-positive or Gram-negative bacteria, depending on light irradiation. Gram-positive bacteria have a membrane composed mainly of peptidoglycans, while Gram-negative bacteria have both an inner and outer membrane made mainly of phospholipids with a thin peptidoglycan layer. This dictates the selectivity towards the photo-switchable ligand functionalised nano-antibiotics.
To be effective against both, the team designed a functionalised nanomaterial of molybdenum disulfide (MoS2) with azobenzene moieties to which they attached positively charged quaternary amino groups. MoS2 is a bactericide, the amino groups facilitate membrane depolarisation, and the azobenzene moieties introduce the light-driven switch in the nanostructure from an elongated trans into a curved cis form.
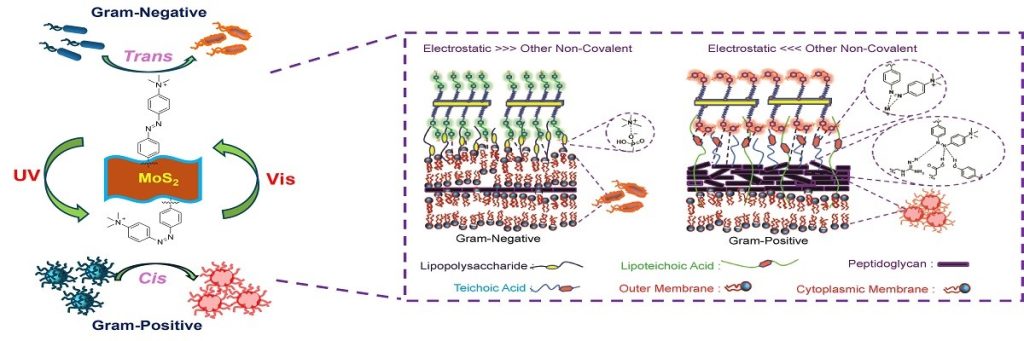
The team used a variety of chemical probes and optical measurements to determine that the cis form is effective against Gram-positive bacteria, whereas the transforms of the nanomaterial were effective against Gram-negative bacteria.
They found that for the Gram-negative P. aeruginosa, the trans form pierced through the bacterial membrane, allowing the MoS2 nanomaterial to generate reactive oxygen species and kill the bacteria. Meanwhile, the cis form was found to be more effective against the Gram-positive MRSA strain through membrane depolarisation. By ‘flipping’ the UV switch from the trans ground state to the cis state, the team was able to use their nanomaterial flexibly to kill different bacteria.
The researchers demonstrated the efficacy of their approach by successfully healing MRSA-infected wounds in mice models at a faster rate when compared to commonly used antibiotics.