Chemical proxies have provided scientists a handle on understanding the planet’s changing climate

Over the last century, scientists have been keenly invested in quantifying the global impact of human activities on the environment. Of key concern is the reconstruction of the climate prior to large-scale human interference. This is where chemical proxies – such as isotopes – come in handy.
Isotopes are atoms of the same element with the same atomic number but a different mass number, owing to the different number of neutrons in their nuclei. Due to their differing properties in different environmental conditions, they have become an essential part of a climatologist’s toolkit to understand the past and predict the future weather of Earth and other planetary bodies. Tiny differences in the stable isotope ratios in biological and abiotic archives have revealed more about the Earth’s climate than most other methods. Perhaps the best example of this is the change in the ratio of carbon isotopes (¹³C/¹²C) present in the atmosphere. Since the Industrial Revolution in the mid-19th century, when humans started burning fossil fuels on a massive scale, there has been a significant decline in the atmospheric ¹³C/¹²C ratio. Plants prefer absorbing ¹²C during photosynthesis, and increased human dependence on this fossilised plant matter has released tremendous amounts of ¹²C in the atmosphere.
Several PhD students at the Centre for Earth Sciences (CEaS) and the Divecha Centre for Climate Change (DCCC) at the Indian Institute of Science (IISc) are also harnessing the potential of isotopic signatures to answer questions about our planet’s past as well as the present.
Rachita Ghosh is studying ancient rock exposures from Badami, Karnataka, in order to reconstruct the climate in the past (palaeoclimate). This sedimentary rock locality that she focuses on primarily comprises limestone, dolostone and carbon-rich shale. Limestone and dolostone are made of calcite and dolomite respectively. The premise behind her work is simple. Lighter isotopes are always the first to move from the stable phase (here, liquid) to the unstable phase (here, gas). Rising surface temperatures lead to the evaporation of ocean water, and water molecules containing lighter oxygen isotopes evaporate faster. As a result, the water that is left is rich in heavier isotopes. And so, a carbonate rock with a greater quantity of heavier isotopes of oxygen indicates that the rock was formed or precipitated in warmer waters.
The same principles can be applied to other elements and other natural archives that capture these processes. Neha Tanwar is studying how the magnesium isotope ratios have changed in seawater over the last 70 million years as recorded in fossil foraminifera shells. Foraminifera are single-celled microorganisms that secrete a tiny shell made from calcium carbonate around themselves. Since each species dwells in different salinity and temperature conditions, and in different “habitats” (soil, mud, weed), their shells can be used to determine the age and environment in which foraminifera grew. The Mg/Ca ratio in foraminifera shells is used to determine the temperature of the aquatic environment in which they were formed.
On Earth and beyond
Each isotope gives a unique glimpse into Earth’s history. This has enabled Utpalendu Haldar to see how the chemical composition of Earth’s crust changed over time. He focuses on neodymium isotopes, which are released from rocks into surface waters by the erosion of the continental crust. As they carry the neodymium signature from nearby continents, marine archives offer an excellent palimpsest of past erosional processes. The movement of glaciers is one such process that cuts through mountains, revealing a record of the geophysical changes.
“Isotopic compositions of natural archives offer a great tool to understand processes going on, both on the Earth’s surface as well as below,” explains Sourav Ganguly. He examines the isotope ratios of strontium and calcium in the Sundarbans to study the movement of water between large rivers, ground water and sea water.
Isotopes can yield information about extraterrestrial phenomena as well. Thamizharasan Sakthivel works on how cosmic rays – high energy protons and nucleons that move as fast as light in space –impacted terrestrial biomes. Examining near-Earth explosions of stars (supernovae)that occurred 1.8 to 3 million years ago, one of his studies showed that this phenomenon triggered worldwide wildfires, effecting a vegetational shift from forests to grassland. “We are not the [first] ones [to have] proposed these hypotheses. These hypotheses have been well played in the hands of physicists since the 20th century but [were] not familiar among geoscientists. We substantiated the astrophysical theory with geological evidence,” he explains.
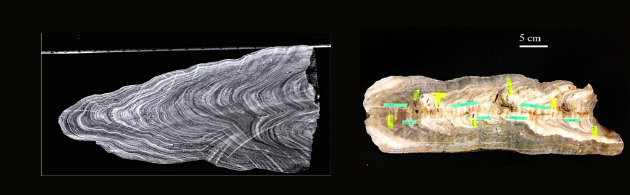
(Image courtesy: Rachana Subba)
Finally, a key question in isotope-based reconstructions of climate change is the precision to which these chemical proxies are able to successfully record changes in parameters such as rainfall and temperature. To this end, Rachana Subba works on speleothems, which occur in caves and are formed as calcium carbonate aggregates over time, very much like ice cores and lake sediment cores. There are existing records of rainfall for the recent past, and Rachana is using chemical signatures found in speleothems as an analogy for rainfall. “The speleothem records form layer-by-layer, have a good resolution, sometimes at sub-annual levels as well,” she explains. “Indian caves, having been subject to both south-west and north-east monsoon rains, serve as a natural rain gauge, helping detect past climate well before humans started measuring rainfall.”
Harnessing the potential of carbon and oxygen isotopes, Bhanu Priya Thakur aims to reconstruct the palaeoclimate by tapping into vertebrate fossils, such as teeth or bones. Teeth offer a distinct advantage to such studies because of their preservation and abundance in the fossil record. Explaining the relevance of her work, she says that the Cretaceous Period (145-65 million years ago), was a fairly warm period in Earth’s history. “The period I am examining was quite similar to future climate projections, and my studies could inform how biomes and their inhabitant communities are likely to shift should these projections become a reality,” Bhanu explains.
Because they are highly sensitive to changes in water, land and air, isotopes serve as excellent indicators of ecological changes. However, the future holds some challenges for isotope research. Sampling of materials has to be well distributed across geography and time, in order to establish solid parameters that future isotope-based observations can be based on. Museums are also rapidly decommissioning their natural archives, which have served as a goldmine for all isotope researchers, making the hunt for isotopes a real race against time.