The rise of multi-drug-resistant Staphylococcus aureus has become a global health concern, with a toxin called alpha-haemolysin (α-HL) playing a central role in the bacterium’s pathogenesis. Despite extensive studies, most structural analyses of α-HL have been performed using detergents or one lipid moiety, which do not reflect the complexity of natural cell membranes.
In a new study, researchers at the Molecular Biophysics Unit led by Somnath Dutta sought to understand how α-HL behaves in a physiologically relevant lipid environment. Real membranes consist of diverse lipids with varying chain lengths, fluidity, and rigidity – factors that could influence toxin function. However, little was known about how these properties affect α-HL oligomerisation and pore formation.
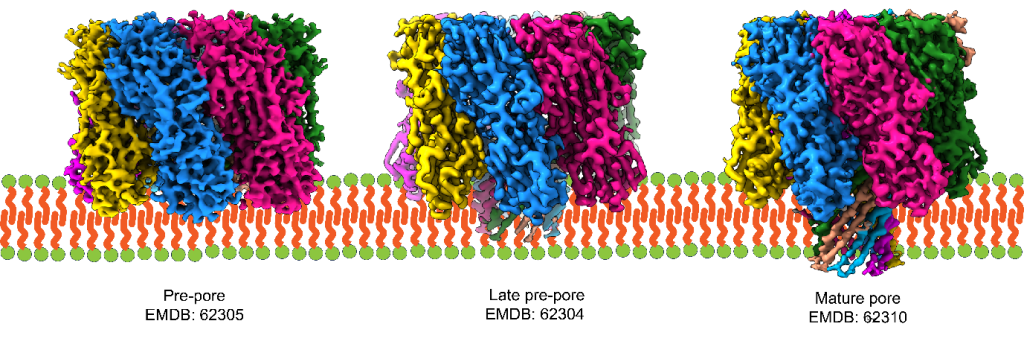
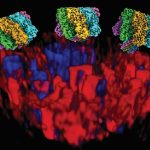
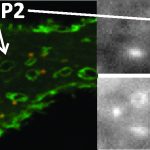
The team used a combination of fluorescence microscopy, single-molecule imaging, negative-stain TEM, mutagenesis, and cryo-EM to study α-HL interactions with different lipid compositions. Their structural and biophysical studies revealed that membranes with shorter lipid chains promote pre-pore formation and membrane rupture, while longer chain lipids support the progression to complete pore structures. Notably, sphingomyelin-rich membranes, due to their rigidity, significantly reduced pore formation.
For the first time, the team demonstrates how lipid composition directly governs the assembly and function of a pore-forming toxin under near-physiological conditions. This understanding provides a new framework to explain the lipid-specific behaviour of bacterial toxins and offers a path forward for designing therapeutic inhibitors.