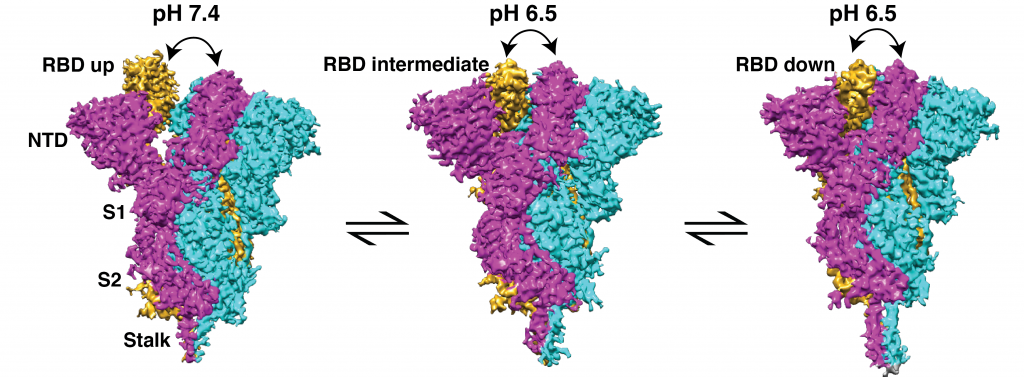
The SARS-CoV-2 S proteins, which appear as crown-like spikes on the viral surface, mediate the entry of the virus into the host cell. They are also the site where neutralising antibodies produced by the host cells bind to the virus to inactivate it. Understanding the protein’s detailed structure is therefore important.
Most previous studies on the S protein structure have been carried out either at pH 8.0 or pH 4.0 to pH 5.0. But the structure of the S protein at physiologically relevant conditions – at which the virus actually infects the host cells – remains poorly understood.
Researchers in the Molecular Biophysics Unit led by Somnath Dutta have now successfully visualised the different conformations or forms of the S protein at physiological pH (7.4) and near physiological pH (6.5 and 8.0) using a technique called single-particle cryo-electron microscopy.
The team observed that around 68% of the S proteins exist in open conformation at physiological pH 7.4, but their proportion decreases when the pH is slightly higher or lower. They also detected many intermediate conformations between fully open and closed, and showed that distinct states of both conformations have different binding affinities towards neutralising antibodies.