An IISc team has synthesised a nanozyme that can break up the cell membranes of pathogenic bacteria and reduce biofilm formation in urinary catheters
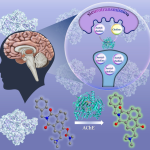
In a significant breakthrough in the battle against antibiotic resistance, a research team from IISc has synthesised a nanomaterial that mimics an enzyme and can disintegrate the cell membranes of a range of disease-causing bacteria. The study, published in the journal ACS Applied Bio Materials, is a collaboration between researchers from the Department of Inorganic and Physical Chemistry (IPC) and the Department of Microbiology and Cell Biology (MCB).
The discovery of antibiotics revolutionised the field of medicine. By the 1960s, many health experts even believed that the fight against infectious diseases was in its final stages. However, recent decades have seen a new challenge – the evolution of resistance to antibiotics in pathogenic bacteria.
Antibiotics typically work by interfering with the cellular activities of the bacteria. Over many generations, owing largely to the misuse and overuse of antibiotics, several strains of bacteria have developed resistance to antibiotics by producing their own enzymes that target the drugs.
The cell membranes of all organisms, including bacteria, have two layers of lipids containing phosphate molecules. “Phospholipid is an essential component of the cell membrane,” explains Kapudeep Karmakar, a former PhD student at MCB and the joint first author on this paper along with Kritika Khulbe, former PhD student at IPC.
Therefore, the researchers decided to target these phospholipids with the help of nanomaterials that would break the bonds holding the membrane bilayer together. These nanomaterials are known as nanozymes. According to the authors, since the nanozymes directly target the chemical integrity of the phospholipids to destroy the cell membrane, bacteria are less likely to be able to develop resistance against them.
To develop this novel compound, the team synthesised a cerium oxide-based nanozyme using what is known as a chemical co-precipitation method. In the next step, they carried out a reaction between cerium oxide and sodium polyacrylate in a basic solution to coat the nanoparticles with polymers. The polymer coating allows the nanozyme to disperse onto any surface or material and boosts its activity.
The nanomaterial was then tested in the lab on several potentially pathogenic bacteria such as Salmonella Typhi, Shigella flexneri, Escherichia coli, Vibrio cholerae and Klebsiella pneumoniae, which cause typhoid, gastroenteritis, dysentery, cholera and pneumonia respectively.
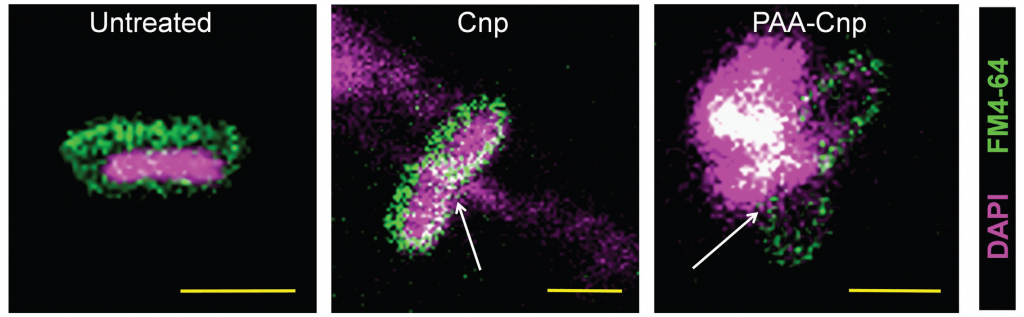
What the team found was that the nanozyme stopped their growth and subsequently inhibited the formation of biofilm – a densely packed community of bacteria.
“Most antibiotics are not able to penetrate through biofilms. Our nanomaterials were able to penetrate even a 10-day old, well-developed biofilm and showed anti-bacterial activity inside the biofilm because of their small size,” says Khulbe.
The researchers also tested the nanozyme on urinary catheters. These medical devices are vulnerable to formation of pathogenic biofilm on their surfaces, leading to infections in patients. In a laboratory setting, the team found that the bacterial attachment to the catheter surface significantly reduces on treatment with the nanozyme. Because the nanozyme does not distinguish between human and microbial cells, the researchers strategically coated only the inner surface of the catheter to kill the microbes. In order to use their nanomaterial in other medical devices, more research would be required to ensure that there is no contact between human cells and the nanozymes.